A team of Canadian researchers has shed new light on Amyotrophic Lateral Sclerosis, an illness which gradually paralyses muscles and currently has no effective cure. Hope for new drugs.
In the many and varied studies which are finally yielding some results (albeit limited, for the time being) on the therapeutic management of an illness as difficult as Amyotrophic Lateral Sclerosis (also known as Motor Neurone Disease and Lou Gehrig’s disease), alterations in certain “mechanisms” have been flagged in a gene called C9orf72, and the important role it plays in the onset of this illness.
And we all know that casting light on the actual genetic causes of an illness can also help to find a remedy.
The study, which was funded by ALS Society of Canada and other Canadian foundations, was conducted by researchers from the Institut national de la recherche scientifique (INRS) and published in Communications Biology (of the Nature group).
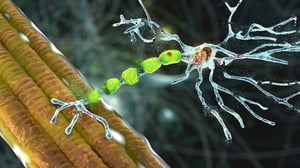
ALS is a neurodegenerative disease caused by the gradual destruction of the brain’s motor neurones and spinal bone marrow (the nervous cells which govern muscles).
Over the years, ALS triggers paralysis of voluntary muscles and eventually spreads to respiratory muscles as well.
To date there are no effective pharmacological cures for this illness, but research is making rapid progress. Average survival rates have improved in recent years thanks to improved clinical management of patients, and the use of increasingly sophisticated equipment.
As stated before, there is no real treatment as such. But recent months have seen the arrival of encouraging signals from the “CENTAUR” study published in the New England Journal of Medicine, revealing the ability of two “associated” molecules, sodium phenylbutyrate and taurursodiol, to slow the disease down.
Details of the discovery
But let’s first go back to the study just published in Communications Biology.
Working on animal models (tiny genetically-modified zebra fish), the Canadian researchers at the INRS demonstrated that one particular mutation in the C9orf72 gene (already pinpointed as a possible “protagonist” of ALS some time ago) is actually linked to the damage typically caused by the illness. The experts also managed to describe in some detail how this mutation interferes with the normal transmission of nervous impulses to the muscles.
The researchers discovered that the problems are triggered by an anomalous succession in the nitrogenous bases (known as the “building blocks” of DNA) within gene C9orf72: in particular, many people with ALS present an extreme multiplication (a thousand times instead of the normal 20) of a sequence indicated as the GGGGCC repeat (G being the code for guanine and C that of cytosine, two of the nitrogenous bases of DNA. The other two are adenine and thymine).
The researchers claim that this genetic disorder is what is altering the activity of the C9orf72 gene, causing a loss of function (as it is termed in the business) as well as having a negative bearing on communication between the motor neurones and muscles. The mutation is present in 40-50% of people with a hereditary form of ALS, and in 5-10% of those who have a non-familiar form of the illness.
The mutations of gene C9orf72 also play a part in frontotemporal dementia (FTD), a form of dementia which differs from Alzheimer’s, and affects around 15% of ALS patients.
Consequences also on another gene
According to the researchers, the alteration of the C9orf72 gene also has a knock-on effect on another gene called TDP-43 (transactive response DNA-binding protein 43), which is crucial for correct replication of DNA.
Usually, this second gene encodes a protein which remains in the nucleus, where it fulfils its role during DNA replication, but in 97% of patients is located outside the nucleus, in the cytoplasm, where it forms aggregates whose purpose is as yet little understood, and the effects of which will be analysed in forthcoming studies.
Deciphering such a crucial, well-documented phase of an illness which to date has proven tough to crack might finally make it possible to achieve significant progress in early diagnosis (by checking for the presence of mutated genes) and in therapy (by correcting the outcome of the mutations).